Researchers offer insights into aging
 UGA scientists have presented a strong case that the mechanism lies at the intersection of the genome and epigenome
UGA scientists have presented a strong case that the mechanism lies at the intersection of the genome and epigenome
What determines the life span of a mouse, alligator, dog or human? A team of scientists at the University of Georgia believes they have new insight into this age-old question.
Emily Bertucci and Benjamin B. Parrott, a research team at the Odum School of Ecology and the Savannah River Ecology Laboratory, have presented a strong case that the mechanism lies at the intersection of the genome and epigenome.
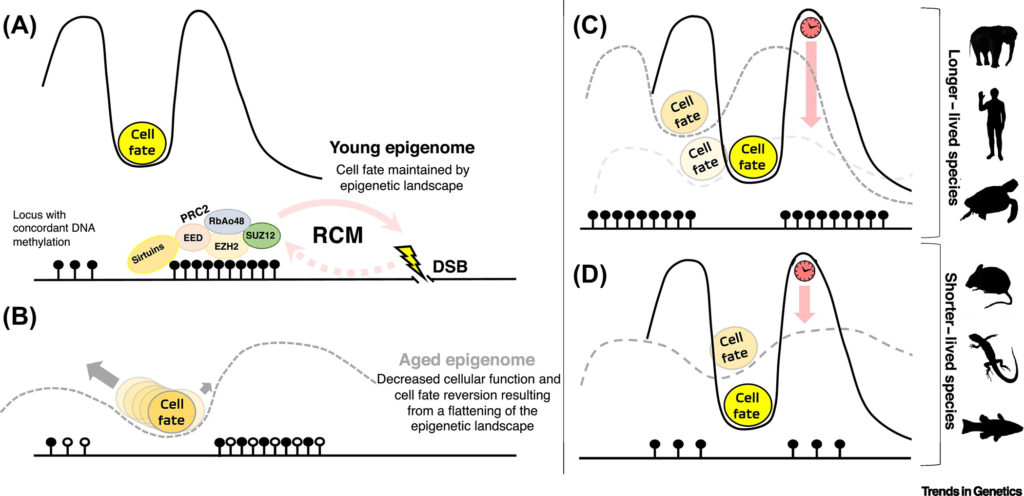
Bertucci, a doctoral candidate, and Parrott, an assistant professor, build on recently published data in epigenetics, which underlies changes in organisms caused by modifications to gene expression rather than alterations in genetic code. Their recent literature review, published in the journal Trends in Genetics, reports that a significant part of epigenetic aging occurs in regions with CpGs. A CpG is a region of DNA where the chemical elements cytosine and guanine are connected by a phosphate bond.
“CpGs are important because DNA methylation occurs in the area,” said Bertucci, the study’s lead author. “Methylation adds a chemical code to the DNA strand that empowers the strand and tells it how to function. It can also turn genes on or off.”
Scientists have observed that methylation patterns change as vertebrates age.
“We know that certain changes occur in the genome at certain times. These changes can be modeled to create epigenetic clocks and occur in the region associated with developmental genes,” he said. “You can take a blood sample to sequence DNA methylation and accurately predict age within several years.”
The activity that occurs in this area also determines a cell’s fate—whether it will be a skin cell, neuron or a heart cell—but this is just one piece of the puzzle.
Parrott said double-stranded breaks frequently occur in the genome throughout an organism’s life span. These breaks are efficiently repaired by proteins and enzymes known as chromatin modifiers.
According to the study, the chromatin modifiers leave the CpG Island to repair the breaks, and when they relocate, changes occur that alter gene expression and thus a cell’s fate.
If we think of these chromatin modifiers as soldiers, their role becomes quite clear, Bertucci said.
“They have unique power to support and enforce the code of the land, but if they leave the island to perform a rescue, the island becomes compromised and vulnerable if all of them don’t return.”
Scientists have known for years that species with longer life spans such as humans, alligators and elephants have higher levels of CpGs than species like fish and rodents, Parrott said. He likens increased CpGs on the island to a bright light beaconing from a lighthouse that aids the relocated chromatin modifiers or RCMs to make their way back home safely. They also act as a buffer to keep the foundation of the epigenetic landscape intact when the RCMs move to repair the double-stranded breaks.
The density of CpGs may not offer the total picture of life span, according to the researchers. Environmental factors and stress can also play a role.
The details of the study, including a list of the research the team reviewed, can be found at https://www.sciencedirect.com/science/article/pii/S0168952520301323
